Abstract
Background Relapsed acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) are associated with a poor prognosis. It is not known which re-induction therapy provides the highest chance of durable remission. Commonly used re-induction therapies are high dose cytarabine (HiDAC) and triple therapy consisting of fludarabine, cytarabine, idarubicin combined with granulocyte-colony stimulating factor (FLAG-IDA). Re-induction is mostly followed by consolidation therapy consisting of allogeneic stem cell transplantation (SCT) or donor lymphocyte infusion (DLI). In this study, we retrospectively compared the outcomes of HiDAC and FLAG-IDA in patients with relapsed AML or MDS.
Patients and methods Two patient cohorts with relapsed AML or MDS treated between October 2015 and December 2021 in two different academic hospitals (Academic Medical Center (A) and VU Medical Center (B)) in Amsterdam, The Netherlands, were compared. Patients at center A were treated with HiDAC, while those at center B were treated with FLAG-IDA as re-induction therapy. Clinical and laboratory data were systematically collected and retrospectively analysed. Outcome measures, including complete remission (CR), overall survival (OS) and event-free survival (EFS; defined as survival until refractory disease, relapse or death), were compared between both cohorts.
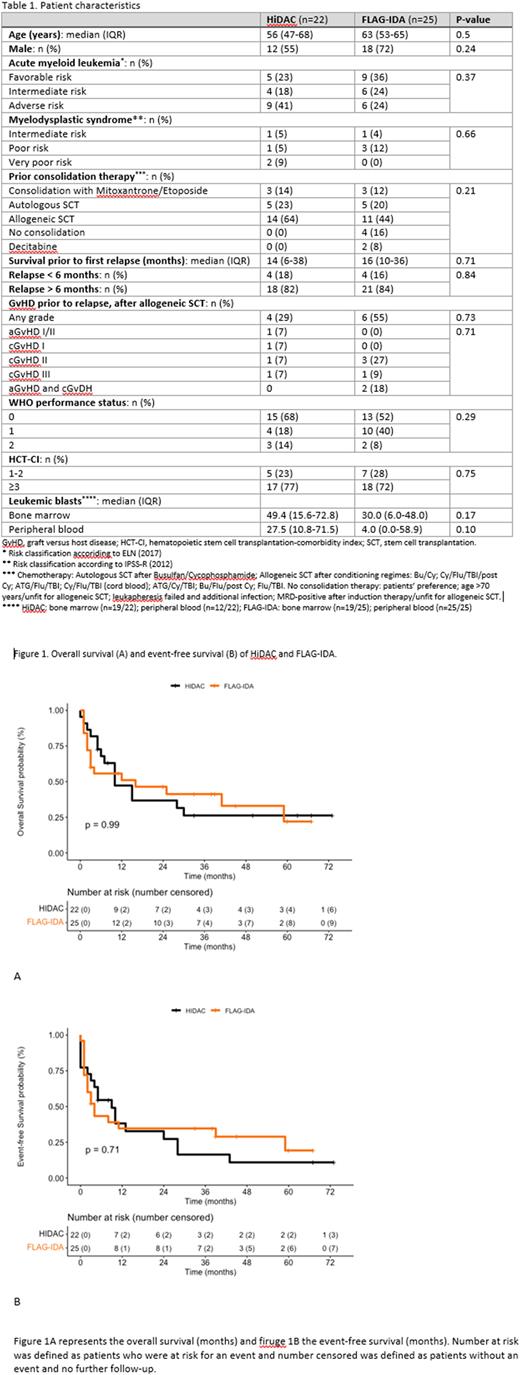
Results A total of 47 adult patients with a relapsed AML or MDS had been treated with either HiDAC (n=22) or FLAG-IDA (n=25). The two groups did not differ in age, sex or risk classification (see Table 1). In the HiDAC group, 64% of patients had previously undergone an allogeneic SCT compared to 44% in the FLAG-IDA group. HiDAC and FLAG-IDA resulted in comparable rates of CR (71% vs. 74%, P=0.85), 1-year OS (47% vs. 51%, P=0.99, see Figure 1A) and EFS (38% vs. 35%, P=0.71, see Figure 1B). The proportions of patients receiving consolidation treatment (allogeneic SCT or DLI) after re-induction were also comparable between the groups (64% vs. 60%, P=0.42). Patients who received HiDAC had significantly shorter durations of neutropenia and thrombocytopenia compared to patients treated with FLAG-IDA (median 24 days (IQR 20-26) vs. 30 days (22-39), P=0.014, and 22 days (17-26) vs. 36 days (26-53), P<0.001 respectively). The number of transfused pools of thrombocytes was also significantly lower in patients treated with HiDAC compared to those treated with FLAG-IDA (9 (6-14) vs. 12 (9-21), P=0.046). The number of transfused units of red blood cells was significantly higher in the FLAG-IDA group. However, since patients were kept on a higher hemoglobin level at center B than center A, we did not include this comparison in our analysis. The incidence of adverse events was similar in the two treatment groups.
Conclusion While remission rates and survival outcomes were similar between both groups, FLAG-IDA was associated with longer periods of myelosuppression and transfusion dependency compared to HIDAC. Although limited by the retrospective design and relative small number of patients, these results favor HiDAC over FLAG-IDA as re-induction therapy for relapsed AML or MDS.
Disclosures
van de Loosdrecht:Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Alexion: Membership on an entity's Board of Directors or advisory committees. Wondergem:Novartis: Other: Steering Committee; BMS, Sanofi: Other: Educational talks. Janssen:Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Speakers Bureau; Ellipses Pharma: Research Funding; Avillion: Research Funding; Glycomimetics: Research Funding; Uppsala County Council: Research Funding; Pfizer: Consultancy; Incyte Biosciences Benelux BV: Research Funding, Speakers Bureau; Novartis: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding. Zweegman:Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Biemond:Modus Therapeutics: Membership on an entity's Board of Directors or advisory committees; Chiesi: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; GBT: Research Funding; BMS: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Chiesi: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; GBT: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanquin: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. de Leeuw:Takeda: Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria; AbbVie: Consultancy, Honoraria. Nur:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal